Introduction: Acute graft versus host disease (aGVHD) carries high morbidity and mortality after hematopoietic stem cell transplantation (HSCT). Corticosteroids remain the mainstay treatment approach for aGVHD, with limited efficacy (≤50%). Different treatment modalities have been studied to improve outcomes. Monoclonal antibodies (mAb) are currently being tested, especially for steroid-refractory (SR) aGVHD.
Methods: A comprehensive literature search, from 2015 to 2020, was performed using Medline, Embase, and Cochrane. MeSH terms and keywords were HSCT, acute GVHD, and monoclonal antibodies. PRISMA guideline was used for study selection.
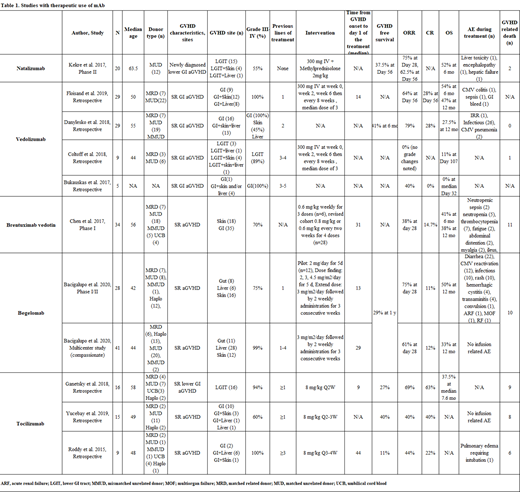
Results: A total of 11 studies were identified, including 241 patients. Extracted outcomes were depicted in Table 1. Ten studies included patients with SR aGVHD (n=221), with most of them having GI involvement. Most of the patients had ≥grade III-IV aGVHD and concomitant organ involvement. Most of the studies continued systemic steroids and gradually tapered based on the therapy responses.
Natalizumab: In a phase II trial, 20 patients with newly diagnosed lower GI aGVHD received natalizumab along with methylprednisone. 55% of the patients had grade III-IV. The overall response rate (ORR) was 62% at day 56, and overall survival (OS) was 52% at six months. GVHD free survival was 37.5% on day 56, and two patients died from GVHD.
Vedolizumab:
Four studies evaluated vedolizumab in SR GI aGVHD patients (grade ≥III-IV). In a study of 29 patients, durable responses were reported; ORR and complete response (CR) were 64% and 28% at day 56, respectively. Overall survival (OS)was 47% at 12 months. In this study, patients received vedolizumab as a 2nd line. Another study, n= 29 patients, also showed positive results with ORR of 79% with CR 28%. OS at 12 months was 27.5%. The other two small studies, however, reported low response rates with high mortality. Remarkably, in these studies, vedolizumab was given as a 4th or 5th line.
Brentuximab vedotin: Ina phase I study, n= 34 SR aGVHD patients (70% grade ≥III-IV) demonstrated ORR of 38% at day 28. OS was 41% at six months, and 38% at 12 months. In this study, dose revision was made (from 0.6 mg/kg weekly to 0.6-0.8 mg/kg every-2-weekly) after deaths due to neutropenic sepsis.
Begelomab: A phase I/II study evaluated 28 patients with SR aGVHD. All patients had gut, skin, and liver involvement before the therapy (75% grade III-IV).ORR at day 28 was 75%, CR 11%, and OS was 50% at 12 months. Patients received begelomab within the 13 days from the diagnosis of GVHD after failing one previous line. A compassionate use study including 41 patients demonstrated ORR of 61% on day 28 and CR of 12%. OS at 12 months was 33%. For this group, begelomab was given after 1-4 lines of previous therapies.
Tocilizumab: A study of 16 patients with GI aGVHD (94% of grade ≥III-IV) demonstrated ORR of 69%, CR rate of 62.5%, and median OS of 7.6 months. However, only three patients remained GVHD-free after discontinuation of tocilizumab. In a small retrospective study of 15 patients, 40% of patients achieved CR with a resolution of aGVHD. Patients with liver aGVHD did not respond. A durable response was not achieved for nine patients.
Conclusion:
Monoclonal antibodies for the treatment of aGVHD show encouraging results. Responses appear to be related to the duration and timing of the therapy, GVHD severity, organ involvement, and the number of previous lines of therapy received. Large prospective clinical trials are needed to further solidify the role of monoclonal antibodies in aGVHD treatment.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal